How protein found in human sweat may protect against Lyme disease
It started in Russia “tick season”which means that walking outside now requires us to be especially careful.
Every year in Russia about !half a million people! remove the tick from yourself at least once. Many incidents end calmly and without serious consequences, but some inevitably lead to a hospital bed. Ticks carry a number of serious diseases that can be transmitted to humans through their bites. These include: Lyme disease (borreliosis), tick-borne encephalitis, ehrlichiosis, tick-borne rubella etc.
The most famous disease is borreliosis. It is dangerous because some patients are left without effective treatment, due to lack of effect from antibiotics.
In this article I will talk about how scientists managed to discover a protein produced in sweat that can inhibit the growth of bacteria that cause Lyme disease.
Happy reading 🙂
Introduction to the Study
Lyme disease is a tick-borne infectious disease caused by bacteria of the genus Borrelia. Although many patients are successfully treated with standard antibiotics, there is a risk of developing severe disease leading to chronic complications. Cases of Lyme disease have been increasing in recent years, especially in the northern hemisphere.
Due to the fact that not all risk factors and mechanisms of the disease have yet been studied, the researchers conducted genome-wide association studyusing data from the International Classification of Diseases (ICD), which aimed to identify significant genetic links and better understand the biology of this disease.
Genome-Wide Association Study (GWAS) is a research method aimed at identifying associations between a human gene and specific phenotypic characteristics or diseases.
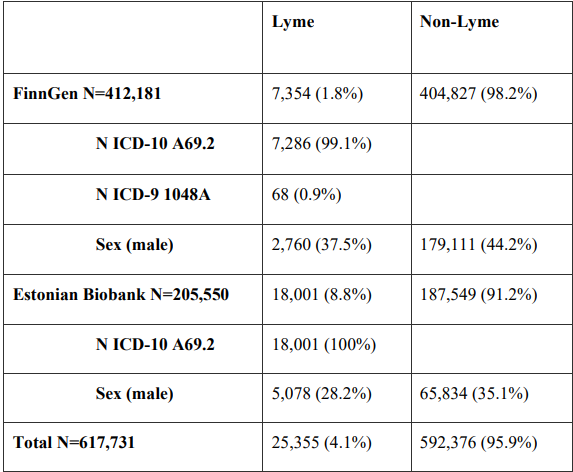
This study analyzed data from 617,731 people, of whom 25,355 had Lyme disease. Diagnosis was based on ICD codes obtained from the national medical registries of Finland and Estonia.
Research results
First, let's look at what GWAS analysis is:
GWAS is a method for identifying genetic variants associated with specific diseases or traits. The process includes the following steps:
Participant Recruitment: Assembling a group of people with the disease and a control group without it.
Genotyping: Analysis of participants' DNA to determine frequencies of single nucleotide polymorphisms (SNPs) (a place in DNA where only one nucleotide differs between people; this is the most common type of genetic difference between people).
Data analysis: Comparison of SNP frequencies between diseased and healthy individuals to identify statistically significant associations.
Confirmation of results: Testing findings using other samples to validate relationships.
Further study: Study the functions and biological pathways of the identified genetic variants.
GWAS help identify which genes may play a role in disease or certain traits.
In summary, GWAS studies related to Lyme disease have identified three genetic regions (loci)which potentially influence the risk of developing the disease.
Genetic loci are specific regions on chromosomes where genes or DNA sequences that have a biological role are located. In the context of genetics, the term “locus” is often used to discuss a specific mutation or variation in a specific DNA sequence.

One of the key loci (expectedly) discovered in this study is in the region human leukocyte antigen – HLA on chromosome sixwhich is traditionally associated with the risk of developing various infectious and autoimmune diseases.
In the course of further research, it was determined that HLA class II lociare associated with a higher risk of Lyme disease, and more specifically HLA-DQB1 gene. It was found to be closest to the areas associated with disease in both study populations. This suggests that HLA-DQB1 may play an important role in the response to the causative agent of Lyme disease, and may contribute to increased susceptibility to this disease.
HLA class II loci are known for presenting antigens to helper T cells, which are important in regulating the immune response.

In addition, the study found a connection with TLR1 receptor genes.
TLR1, or Toll-like receptor 1, is a protein that is involved in the human innate immune system. This receptor is found on the surface of immune cells and helps them recognize specific patterns found in many pathogens, known as pathogen-associated molecular patterns (PAMPs).
TLR1 also cooperates with other similar receptors, such as TLR2, to recognize a wider range of pathogens. Mutations or genetic variations in the TLR1 gene may affect susceptibility to certain infections, including Lyme disease.
Thus, the study showed that Lyme disease may be associated with certain genetic risk factors, supported by associations with HLA and TLR genes.
However the strongest and most meaningful connection was discovered at the gene locus Secretoglobin family 1D member 2 (SCGB1D2). Option rs2232950 this gene showed the most likely causal relationship with Lyme disease and was associated with it in data from both populations.
Secretoglobin family 1D member 2 (SCGB1D2) is a protein belonging to the secretoglobin family that the body normally produces in certain types of tissues, including the glands of the respiratory tract. Secretoglobins are small secreted proteins that may play a role in various processes such as immune response, inflammation, and mucosal defense.
The genetic variant rs2232950 results in an amino acid change in the SCGB1D2 protein, which can distort its structure and interfere with the formation of dimers – structures where two proteins join to perform specific functions in the body.
In addition, the study found that the SCGB1D2 rs2232950 variant is associated not only with Lyme disease, but also with other spirochetal infections.
Spirochetal infections are a group of diseases caused by bacteria from the spirochete class, which includes long, thin, spiral-shaped microorganisms. Such diseases also include: syphilis, leptospirosis, relapsing fever.
As it turned out, the majority of hospitalized patients with spirochete-associated diseases actually had Lyme disease. confirming the special role of the found genetic mutation in the development of this particular disease.
Additional analysis included testing the variant for association with various categories of diseases, such as tick-borne encephalitis, skin infections, syphilis and other bacterial diseases. However, in the end, no significant associations of the SCGB1D2 gene with these diseases were found, highlighting its potential specificity for Lyme disease and uniqueness in the context of other infectious diseases.
So where is the connection with sweat?
The SCGB1D2 protein, which belongs to the secretoglobin family, is produced in the skin and secreted by sweat gland cells. Although the remaining members of this family are mainly found in tissues of the upper respiratory tract, using data GTEx projectit was found that SCGB1D2 is most abundantly produced in the skin, regardless of sun exposure.
*The Genotype-Tissue Expression (GTEx) Project is a broad research initiative that aims to improve our understanding of the genetic regulation of gene expression in various tissues of the human body and its impact on health and disease.
Given that the skin is the first barrier to Borrelia exposure, it was important to know the distribution of SCGB1D2 across skin cell types. A review of single-cell sequencing data showed that SCGB1D2 expression is specific to sweat gland cells. This suggests that SCGB1D2 may be released to the skin surface through sweat. Given that antimicrobial peptides have already been found in human sweat, there is a possibility that SCGB1D2 also has antimicrobial properties.
Does it suppress growth? Borrelia burgdorferi SCGB1D2?
Research Shows SCGB1D2 Protein May Inhibit Growth bacteria Borrelia burgdorferi (Bb)the causative agent of Lyme disease.
Experiments using recombinant SCGB1D2 protein confirmed this effect: at concentrations of 8 and 16 μg/ml, significant inhibition of bacterial growth was observed, and the effect was dose-dependent and persisted for 72 hours.
It is also noticeable that the presence of the protein reduced the number of bacteria in the studied medium, which may indicate not only the suppression of their growth, but also the possible bactericidal effect of SCGB1D2. Although the exact mechanism of action of SCGB1D2 against Bb remains to be determined, the observations indicate the promise of further studies of this protein as a potential antimicrobial agent.
How SCGB1D2 prophylaxis prevents intradermal Bb infection in vivo
To test the effect of SCGB1D2 on the possibility of tick-borne borreliosis infection in a living organism, the scientists conducted experiments by injecting mice with Borrelia burgdorferi (Bb) bacteria treated with the SCGB1D2 protein. Another secretoglobin family protein, SCGB3A1, which is not associated with Lyme disease, was used as a control. The bacteria have been genetically modified to express luciferase.
Luciferase is a protein that can glow under certain circumstances. Thanks to this change, scientists can use special equipment called a real-time imaging system (in this context, In Vivo Imaging System, or IVIS), which detects the light produced by luciferase, to see where genetically modified bacteria are located and how they spread in the animal's body directly. during the experiment.
The results showed that pretreatment of Borrelia bacteria with SCGB1D2 protein prevented the development of infection: there was a significant decrease in the number of bacteria at the injection site compared to the control group. By 10 days after infection, the scientists found no signs of spreading infection in those animals that received Bb, pre-incubated with SCGB1D2.
Thus, SCGB1D2 may have preventive properties against Lyme disease in mice, which opens new prospects for developing methods to prevent this disease.

Conclusion
Currently, many wonderful antibiotics have been discovered and developed, but in almost 50 years of research into Lyme disease, scientists have made little progress. For 10% of those affected, current drugs do not help, and for such patients there is no treatment yet.
Although it is not known exactly how the protein inhibits the growth of the bacteria that causes Lyme disease, researchers hope to use the protein's protective abilities to create skin creams that could help prevent the disease or treat infections that do not respond to antibiotics.
I hope that in the near future, researchers will develop a high-quality medicine that can ensure recovery for the vast majority of those suffering from borreliosis 🙂
You can read the full study at the link: https://www.nature.com/articles/s41467-024-45983-9
That's all!
Thanks for reading 🙂 We'll be waiting for you in the comments!