Who invented the lithium-ion battery
Many tried, but fell out of the race, passing the baton to others, until finally Sony managed to finish the job.
Now, 50 years after the introduction of the first lithium-ion battery, it is easy to talk about its value. It is used in billions of laptops, mobile phones, power tools and cars. Global sales reach $45 billion a year and could exceed $100 billion in the next decade.
And yet this revolutionary invention could not get out of the laboratory for almost two decades: many companies in the US, Europe and Asia studied this technology, but could not unlock its potential.

The first version, developed in 1972 by Stanley Whittingham of Exxon, was not successful: it was produced in small volumes, flashed at the Chicago electric car show in 1977, and was briefly used in watch batteries. Exxon soon closed the project.
Whittingham’s development was picked up by scientists around the world, but for almost 15 years no one has been able to achieve significant results. The path to global domination of lithium-ion batteries only opened when the development finally hit the right company at the right time.
Who first invented the lithium battery?

In the early 1970s, Exxon researchers predicted that world oil production would peak by the year 2000 and then decline steadily. Then the scientists of the company were given the task of finding a replacement for oil, any other way of obtaining energy that is not related to hydrocarbons.
In the fall of 1972, a young British chemist, Stanley Whittingham, joined the Exxon engineering research team in New Jersey. By Christmas, he had developed a battery with a titanium disulfide cathode and a liquid electrolyte that used lithium ions.
Whittingham’s battery was unlike its predecessors. It worked by incorporating ions into the atomic lattice of the electrode material, a process called intercalation. The battery had exceptional characteristics: it was both rechargeable and with a very high energy efficiency. Before that, the best battery was nickel-cadmium, which gave out a maximum of 1.3 volts. And the invention of the chemist Whittingham produced an astonishing 2.4 volts.
In the winter of 1973, Whittingham was called in by company executives to speak at the New York headquarters to members of the Exxon board of directors. “I went there and explained everything in 5, 10 minutes at the most,” Whittingham said in January 2020. “And a week later they said they wanted to invest in it.”
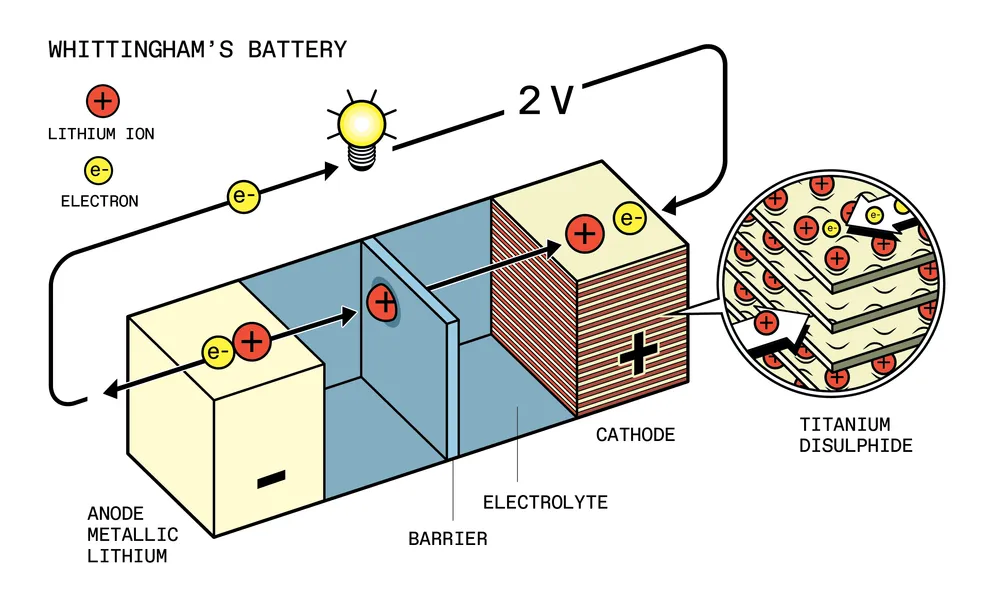
It looked like the start of something big: Whittingham published an article in Science; Exxon started producing lithium disk batteries, and the Swiss watch manufacturer Ebauches began using these batteries in solar-powered wristwatches.
By the late 1970s, however, Exxon’s interest in alternative energy sources waned. What’s more, company executives felt that Whittingham’s development was unlikely to be widely adopted, and washed their hands of it by licensing lithium-titanium battery technology to three battery companies: one in Asia, one in Europe, and one in the US.
“I understood why they did it,” Whittingham says. “The market was just too small. Our invention was ahead of its time.” This was the first of many false starts in the history of lithium batteries.
Oxford takes over

The next scientist to pick up the baton was John Goodenough of the University of Oxford. Goodenough was familiar with Whittingham’s work because Whittingham had received his doctorate from Oxford. But after reading Whittingham’s 1978 article “Chemistry of Intercalation Compounds”, Goodenough was finally convinced that the future belongs to lithium batteries. [Гуденаф скончался 25 июня 2023 г. в возрасте 100 лет.]
Together with researcher Koichi Mizushima, Goodenough began working on batteries based on the lithium intercalation process. By 1980, they had improved upon Whittingham’s idea by replacing titanium disulfide with lithium cobaltite. The use of a new chemical element made it possible to raise the battery voltage by another two-thirds – up to 4 volts.
Goodenough sent letters to battery companies in the US, UK, and mainland Europe in the hope of finding a corporate partner, as he wrote about in his memoir, Witness to Grace. But no one answered.
Then the scientist asked Oxford University for help in paying for a patent, but here he was refused. Like many other universities of that time, Oxford did not deal with intellectual property issues, considering it the lot of commercial organizations.
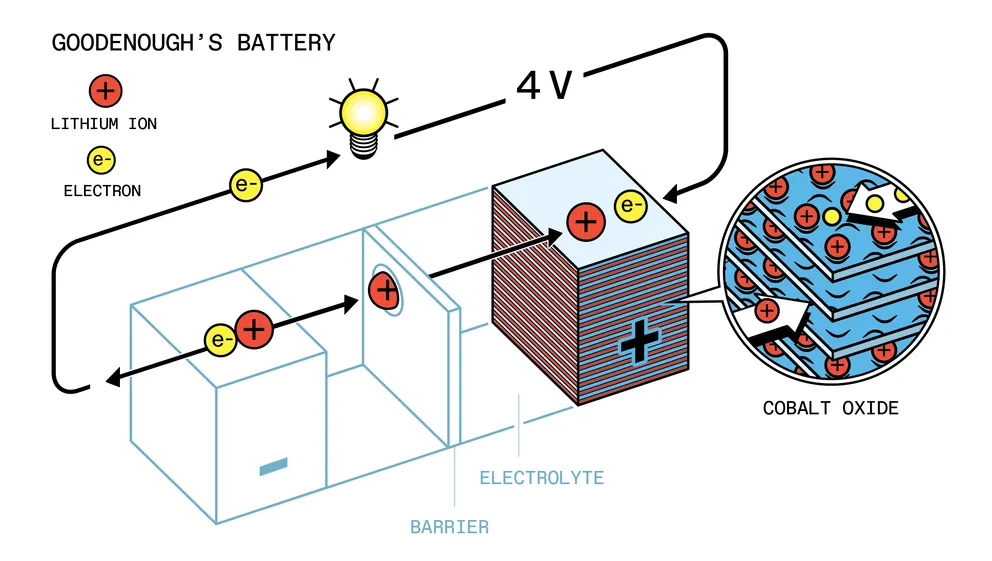
Nevertheless, Goodenough was confident in the correct formula of his accumulator and did not give up. He approached the Atomic Energy Research Establishment (AERE), a government laboratory in Harwell, about 20 km from Oxford. The lab agreed to pay for the patent, but only on the condition that the 59-year-old scientist waive his rights. Goodenough complied. The laboratory patented the battery in 1981, and the scientist was left without a penny of profit from his development.
For the AERE laboratory, this should have been a real gift of fate. Without conducting a single study, she became the owner of an astronomically valuable patent. But the lab managers didn’t see the potential. They filed a patent and forgot about it.
Asahi Chemical comes into play
The next important link in the history of lithium batteries was Akira Yoshino, a 34-year-old chemist at the Japanese company Asahi Chemical. Yoshino had his own development: he was researching the possibility of using a plastic anode made of electrically conductive polyacetylene in batteries and could not find a suitable cathode. In his autobiography, Lithium-ion batteries open the door to the future. Hidden stories of the inventor ”(Lithium-Ion Batteries Open the Door to the Future, Hidden Stories by the Inventor) Yoshino recalled: when he was cleaning up his desk on the last day of 1982, he came across a technical article he had forgotten in 1980, written by co-authored with Goodenough. The article described a lithium-cobalt oxide cathode. Maybe it will fit his plastic anode?
Yoshino, along with a small group of colleagues, connected Goodenough’s cathode to a plastic anode. They also tried combinations with other anodes, mostly different types of carbon. In the end, they settled on a carbon anode made from petroleum coke.
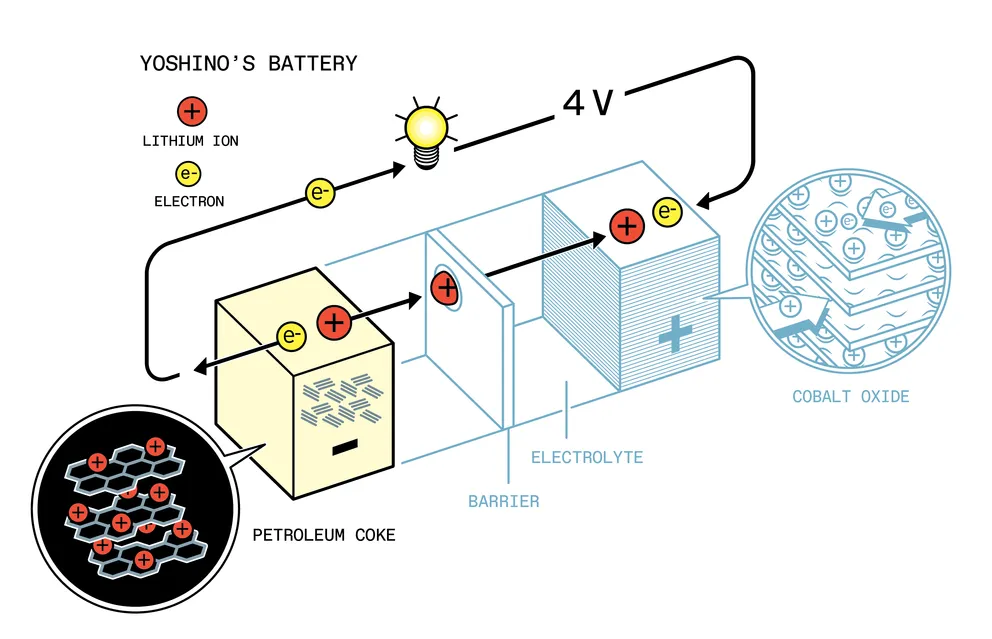
Petroleum coke was a major step forward. Whittingham and Goodenough used lithium metal anodes, which were volatile and even dangerous. By switching to carbon, Yoshino and his colleagues created a much safer battery.
However, not everything went smoothly. Asahi Chemical was primarily a chemical company, not a battery manufacturer. They did not know how to organize production on an industrial scale. Nor did the company have the coating and winding equipment needed to manufacture batteries. The researchers created only a crude laboratory prototype.
Then the head of the research department of Asahi Chemical, Isao Kuribayashi, who participated in the creation of the battery, took up the matter. In his book A Nameless Battery with Untold Stories, Kuribayashi describes how he and a colleague were looking for consultants in the United States to help them manufacture the battery. They were referred to Battery Engineering, a tiny firm based in a converted truck garage in Boston’s Hyde Park neighborhood. The company was run by a small group of scientists. The company specialized in the creation of unusual batteries. They produced a wide variety of batteries for fighter jets, missile silos and drilling rigs.

So Kuribayashi and a colleague flew to Boston in June 1986 and showed up unannounced at Battery Engineering with three cans of slurry: one containing the cathode, one the anode, and one the electrolyte. They asked co-founder Nikola Marincic to turn the cans into standard cylindrical batteries like those used in flashlights.

“They said, if you want to work on our batteries, then don’t ask too many questions,” Marincic said in an interview in 2020. “They didn’t tell me where they were from or why they needed it, but I didn’t ask.”
After Kuribayashi and a colleague also asked Marincic not to tell anyone about their collaboration. Until 2020, even Marincic’s employees did not know that they were involved in the creation of the world’s first pre-series lithium-ion batteries.
For the creation of a batch of batteries, Marincic took $30,000 (today it would be $83,000). And two weeks later, Kuribayashi and a colleague left for Japan with a box containing 200 C-size batteries.
However, even with working batteries on hand, Kuribayashi still met resistance from the directors of Asahi Chemical, who were still afraid to get into an unknown business.
Sony joins the fight
Kuribayashi didn’t want to give up. On January 21, 1987, he visited Sony’s camcorder division to present a new Asahi Chemical battery. He took one of the C-elements he brought from the US and rolled it across the conference room table to his audience.
In his book, Kuribayashi didn’t go into details, saying only that at Sony he only hoped to “validate the new battery technology.”
However, Sony did more than just “confirm”. By this time, according to corporate history, the company was considering developing its own lithium battery. Seeing the Asahi battery, the company immediately realized its great value. Since Sony made both electronics and batteries at the same time, its executives could look at everything from both a customer’s and a supplier’s perspective.
And the timing was perfect. Sony engineers were working on a new camcorder, later called the Handycam, and the product was in desperate need of a smaller, lighter battery. For them, the battery that Kuribayashi introduced seemed like a gift from heaven.
Several meetings followed. According to Kuribayashi, some Sony scientists were allowed into Asahi’s lab, and vice versa. Sony eventually offered to collaborate, but Asahi Chemical refused.
The next part of the lithium-ion battery’s journey to commercial success is murky. Sony researchers continued to work on lithium batteries, using a chemistry that the company’s corporate history later claimed was created in-house. But in fact, it was the same technology as in the Asahi Chemical battery. Lithium cobaltite served as the cathode, petroleum coke served as the anode, and the liquid electrolyte contained lithium ions.
What is clear is that from 1987 to 1989. Sony engineers have done the hard work of turning the prototype into a finished product. Led by engineer Yoshio Nishi, the Sony team worked with suppliers to develop binders, electrolytes, separators and additives. They have developed their own processes for heat treatment of the anode and production of cathode powder in large volumes. Their merit is that they have created a real commercial product.
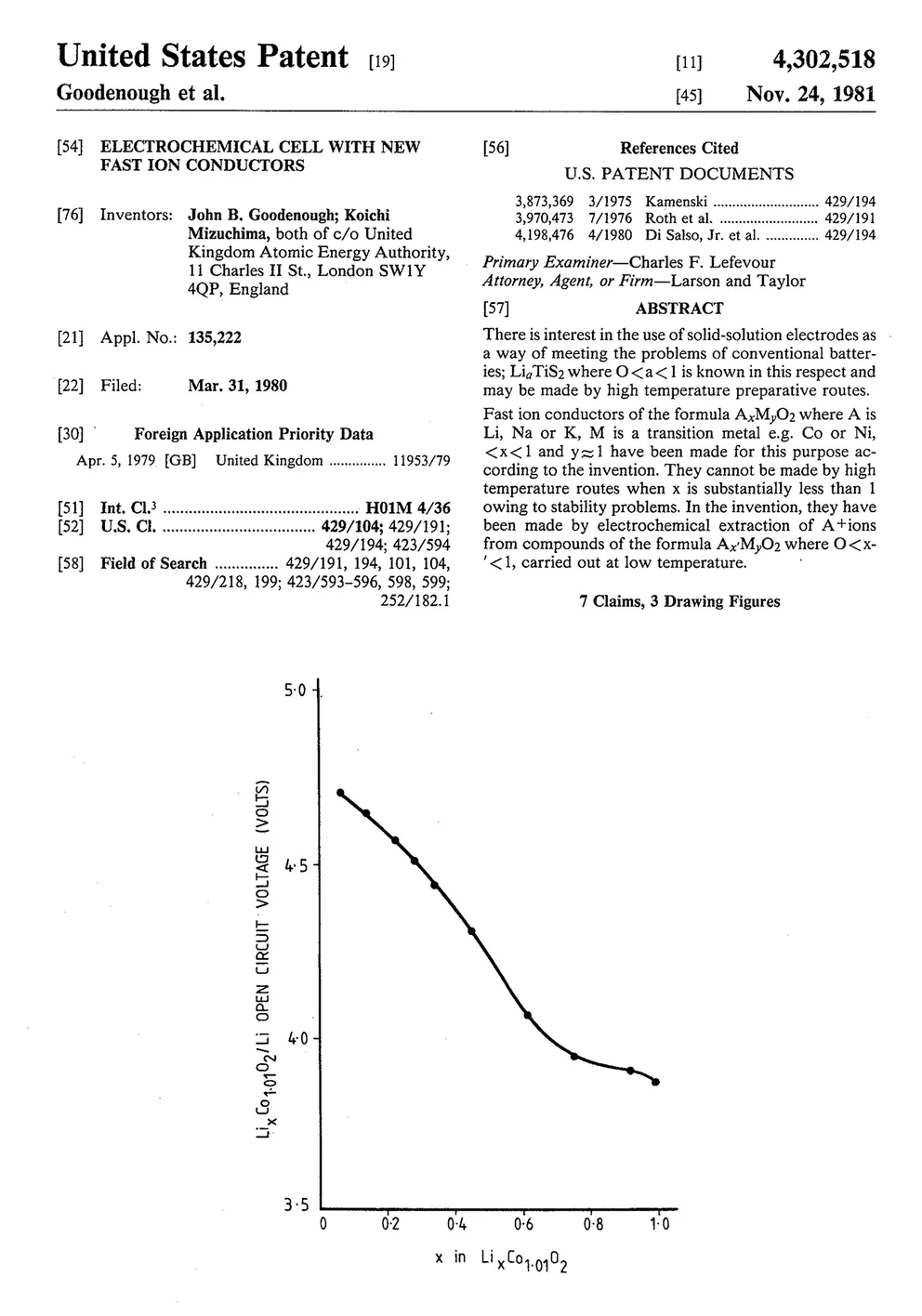
It remained to take the last step. In 1989, a Sony executive called the Atomic Energy Research Establishment in Harwell, England. He asked about one of the lab’s patents that had been sitting idle for eight years, the Goodenough cathode. He said that Sony is interested in licensing this technology.
The scientists and directors of the laboratory at Harwell considered this. They could not understand why someone was suddenly interested in the patent “Electrochemical cell with new fast ionic conductors.” “It wasn’t clear what the market would be, how big it would be,” said Bill McLean, who was a research fellow at AERE at the time. In addition, several older scientists even wondered if it was appropriate for an English atomic laboratory to share its secrets with a company from Japan, with which England fought during World War II.
However, in the end they shook hands.
Sony at the finish line
In 1991, Sony officially introduced a new battery under the now familiar name “lithium-ion”. It immediately began to be used in portable video cameras, and then in mobile phones.
By that time, 19 years had passed since Whittingham’s invention. Several very different organizations had the opportunity to bring this technology to mind, but in the end they abandoned it.
First there was Exxon, whose leaders could not even dream that lithium-ion batteries installed in electric vehicles would seriously compete with oil. Some have since argued that by abandoning the technology, Exxon conspired to eliminate a potential hydrocarbon competitor. However, keep in mind that Exxon licensed the technology to three other companies, and none of them were successful either.
Then there was Oxford University, which refused to pay for the patent.
Finally, there was Asahi Chemical, whose executives took a painfully long time deciding whether to enter a new battery market for them. (Asahi finally got into the game in 1993, teaming up with Toshiba to make lithium-ion batteries.)
Sony and AERE benefited the most from the new batteries. They just got lucky.
Atomic Energy Research Establishment paid only legal fees in exchange for a valuable patent and forgot about it for eight years. AERE’s profit is unknown, but most experts agree that it made at least $50 million, and possibly more than $100 million, before the patent expired.
Fortunately for Sony, Kuribayashi from Asahi Chemical came to them, which eventually led the company to success. Sony sold tens of millions of cells and then sub-licensed the AERE patent to more than two dozen other Asian battery makers, who made billions more. (In 2016, Sony sold its battery business to Murata Manufacturing for 17.5 billion yen, or about $126 million today.)
None of the original authors—Whittingham, Goodenough, and Yoshino—received a share of these profits. However, all three shared the 2019 Nobel Prize in Chemistry. Yoshio Nishi from Sony did not get the Nobel Prize. He criticized this decision.
Now, looking at the history of lithium-ion batteries, we can say that it is like a fairy tale about two worlds. There was a scientific world and a business world, and they rarely overlapped. Chemists, physicists, and materials scientists worked quietly, talking about their triumphs in technical publications and at conferences. The commercial world, meanwhile, was not interested in the breakthroughs of university scientists and did not see the potential of the new battery chemistry. He did not even notice the achievements that were made by his own researchers.
If it weren’t for Sony, lithium batteries could have fallen into oblivion for many more years. It was almost certain that the company was successful because, given the circumstances, it was willing to understand and appreciate Kuribayashi’s prototype. Sony was already in the battery business, they needed a better battery for their new camcorder and they were already thinking about developing their own lithium battery. Sony engineers and managers knew exactly where to put this piece of the puzzle and recognized what many others had overlooked. As Louis Pasteur said more than a century before, “Chance favors the prepared mind.”
The history of the lithium-ion battery shows that Pasteur was right.




