Bacteria in mines: intracellular conversion of copper sulfate to monoatomic copper

Planet Earth is often called the cradle of life, and there is very little exaggeration in this title. Life can be found in the evergreen tropics, and in the sultry deserts, and in the endless ice expanses, and even in the vents of underwater volcanoes. As Ian Malcolm put it in Jurassic Park: “life finds a way.” Scientists from the University of Houston conducted a study on curious bacteria that live in Brazilian mines and can convert copper sulfate ions (CuSOfour) into monoatomic zero-valent copper (Cu0). Why is this process so interesting, and how can it be applied in the human world? We will find answers to these questions in the report of scientists. Go.
Basis of research
It’s hard to imagine the modern world without copper. Dielectric, magnetic, optical, antimicrobial and catalytic properties allow copper to be used in a variety of industries, from solar cells to antimicrobial coatings. Moreover, the chemical synthesis of monoatomic metallic copper is an extremely complex process that requires inert or reducing conditions, as well as the use of toxic reagents. But some organisms are able to do this without difficulty.
Recent studies have shown that many microorganisms, such as bacteria and fungi, can produce inorganic nanoparticles (NPs): Ag, Au, Cu, CuO, magnetite, etc. Particularly curious are the works describing the synthesis of copper nanoparticles in the range from 10 to 40 nm intra- and extracellularly using bacteria. In this case, reductase enzymes such as NADPH (reduced form of nicotinamide adenine dinucleotide phosphate, C21H29N7O17P3), which have a redox potential for the reduction of metal ions. But how microorganisms naturally synthesize CuO (ranging in size from 170 to 179 μm) was previously unclear.
Why is the synthesis of monoatomic copper so attractive? The point is that individual atoms can be used in catalysis, alloying, and power engineering. However, as we already know, monatomic synthesis is associated with a number of difficulties, including the need to use toxic reagents. Alternative methods (vapor deposition, sputtering, and femtosecond laser ablation) are still ineffective.
That is why scientists decided to study in detail the bacteria that independently carry out the monoatomic synthesis of copper without any toxic reagents.
Research results
Bacteria inhabiting the mines of Brazil (6 ° 27′15.848 ″ S and 50 ° 4′37.507 ″ W) were selected as test subjects.

Image No. 1
Finding an isolate of the genus Bacilluscapable of producing monoatomic copper intracellularly was detected by visual observation of color changes in the medium for the growth of bacteria with CuSOfour after 48 hours. Color change from green (CuSOfour + bacteria) orange indicates CuSO transformationfour in Cu0 (1A). Additional confirmation of the presence of Cu0 became the results of transmission electron microscopy (TEM), which shows bacteria after 48 hours of incubation in copper sulfate (1B). On the 1C and 1D shows images of bacteria with higher and lower magnification, respectively. Also on 1D individual copper atoms are clearly visible.
To determine the dimensions of copper atoms, about 13000 atoms (1E). The radius of each atom ranged from 1.7 to 1.85 Å. On the swing chart (1F) summarized the actual distribution of the population and the sizes of all measured atoms detected in the TEM images. More than 75% of copper atoms had a radius of 1.89 ± 0.19 Å. Larger sizes are viewed by scientists as overlapping copper atoms in the images, when one atom is too close to another.
These sizes indicate that the discovered atoms are precisely neutral copper atoms (with zero valence, Cu0). Radii Cu1+, Cu2+ and Cu3+ are in the range from 0.54 to 0.63, i.e. do not fall within the range of observed radii. This additionally confirms that the copper present in the samples is precisely Cu0…
The distribution of elements in cells was determined using energy dispersive spectroscopy (EDS from energy dispersive spectroscopy). The molybdenum mesh ensured that all copper signals detected by the EDS belong exclusively to bacteria (pictures below).
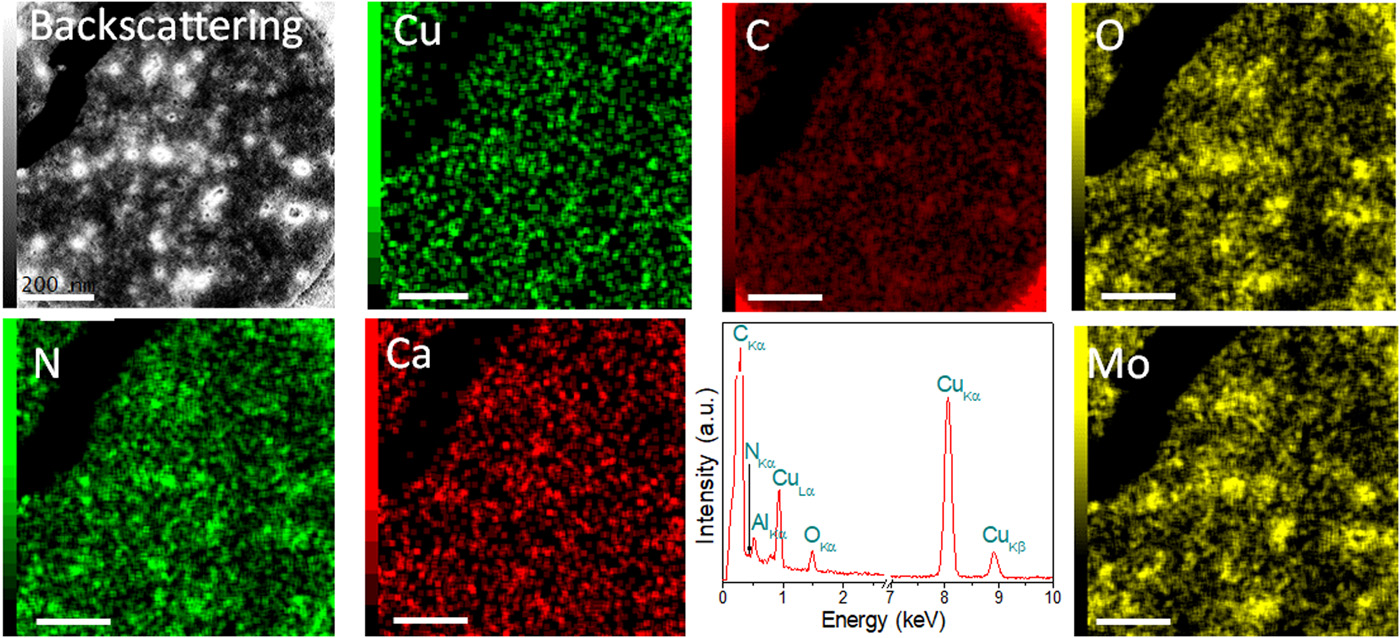
Image No. 2
The backscatter imaging shows a clear contrast between heavier and lighter atoms, in this case between copper and carbon, nitrogen and oxygen.
Consequently, copper is one of the most abundant elements in the sample, and Cu0 – the most common type of copper. This was further confirmed by an analysis of the results of X-ray photoelectron spectroscopy (3A).

Image No. 3
On the charts 3B and 3C shows spectroscopy of characteristic energy losses by electrons (EELS from electron energy loss spectroscopy) copper. In the first EELS spectrum (3B) you can see the presence of carbon, nitrogen and oxygen, which are typical components of organic matter that makes up bacteria. The energy level for each of these elements was: 290 eV – carbon; 400 eV for nitrogen and 530 eV for oxygen.
Copper is usually found in the 931 to 953 eV range. But in this case, it was impossible to detect it using EELS (3C). Scientists attribute this to the thickness of monoatomic copper, which is a known limitation of this method. However, with the help of EELS, it was possible to establish that copper atoms are distributed in cells residually uniformly, and the probability of their clusters is extremely low, in contrast to carbon, nitrogen and oxygen.
On the charts 3D–3F shows the results of X-ray photoelectron spectroscopy of C 1s, O 1s and Cu 2p at the nuclear level, obtained from copper synthesized Bacillus, after 48 hours of incubation.
Spectrum C 1s (3D) represents three components at 285.8, 287, and 288.9 eV, corresponding to the bonds between the protein / peptides and atomic copper. The region of XPS spectra O 1s at the core level (3E) shows the maximum binding energy at 532.8 eV, corresponding to carboxyl groups that belong to proteins on the surface of atomic copper. Cu 2p shows two peaks at 932.3 and 952.0 eV, which correspond to the binding energies of 2p3/2 and 2p1/2 electrons Cu0 (3F). These observations indicate that the type of copper synthesized by bacteria is monoatomic copper, i.e. Cu0…
Having fully made sure that bacteria produce monoatomic copper, scientists need to understand exactly how this happens.
At first, bacterial proteins were identified under two different growth conditions: cultivation without copper sulfate (control group) and with copper sulfate (CuSOfour; 100 mg / l). In the control group, the expression of 652 proteins was recorded, and in the group with CuSOfour – 458 squirrels. Of these 458 proteins, 313 were equally expressed under both growth conditions, and 145 proteins were expressed only in the presence of copper sulfate.
Most proteins (102 proteins) were involved in primary metabolism (carbon and energy). Consequently, Cu affects the cell in a negative way, causing the production of more energy in order to survive this impact. Fifteen proteins were involved in the functions of resistance and stress, and three of them were involved in the transport and uptake of Cu by the cell.
Among the proteins involved in copper transport were the iron-regulated transporter SufB, copper-carrying P-type adenosine triphosphatase (ATPase), and Copz.
Copz is chaperone * a protein that plays the role of intracellular sequestration (accumulation) and transport of Cu + from cytoplasm * in periplasm *…
Chaperones * – proteins that perform the function of restoring the correct native tertiary or quaternary protein structure, as well as the formation and dissociation of protein complexes.
Cytoplasm* – the semi-liquid contents of the cell.
Periplasm * – a separate compartment of cells of gram-negative bacteria.
The remaining 11 proteins identified in the presence of copper sulfate were proteins that can participate in the biosynthesis and stabilization of monoatomic copper. Six of them reduce either sulfate or metals: thiol disulfide isomerase / thioredoxin; thioredoxin reductase (TRXR), dicluster 4Fe-4S, ferredoxin 4Fe-4S, disulfide reductase of the TlpA family and sulfate adenylyltransferase.
Given the functions of these proteins, it can be assumed that they reduce sulfate from CuSOfourleaving free toxic copper (Cu2+) inside the cells.
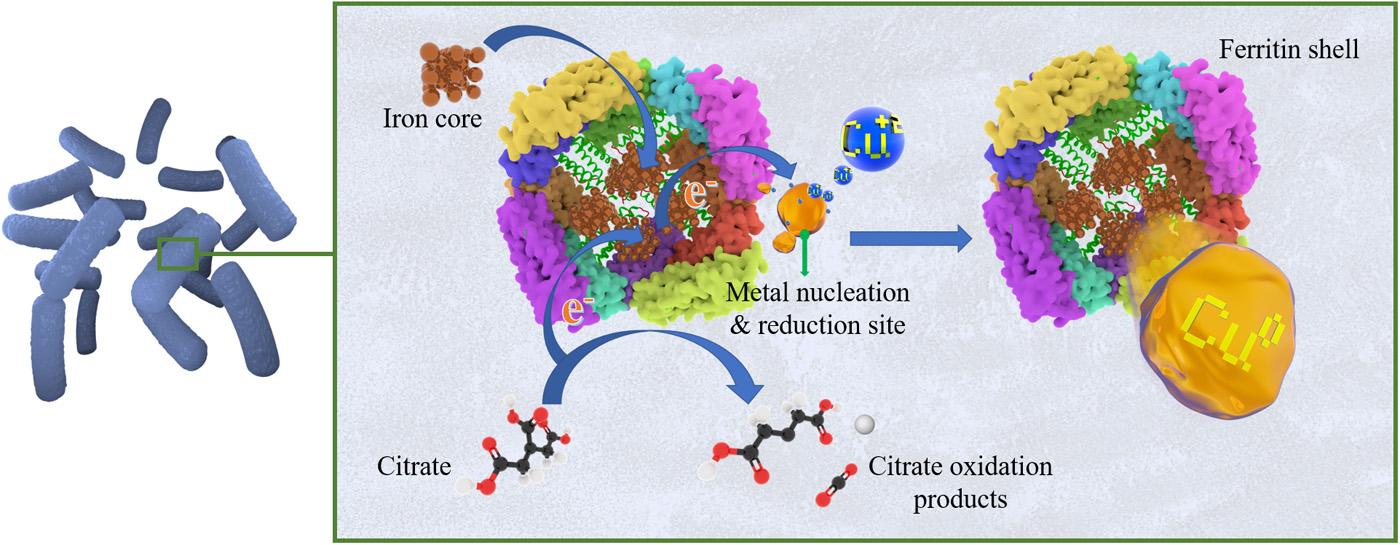
Image No. 4
Production of Se nanoparticles using Stenotrophomonas maltophilia showed a possible association with alcohol dehydrogenase. In this study in bacteria Bacillus Two homologous proteins were identified: NADH (reduced form of nicotinamide adenine dinucleotide) – dependent butanol dehydrogenase A and NADH-dependent butanol dehydrogenase.
This finding indicates that proteins may be involved in the biogenic synthesis of monoatomic copper. Proteins of the Ferritin Dps family and starvation-induced DNA-binding protein (Dps from DNA binding protein). Previously, it was suggested that the pathways for the synthesis of nanoparticles with the participation of these proteins include the processes of autoxidation, hydroxylation, or reduction. But so far this is only a theory.
In this work, scientists suggested that in the process of converting Cu2+ in Cu0 protein plays an important role ferritin *…
Ferritin * – a complex protein complex that plays the role of the main intracellular storage of iron.
It is likely that a combination of this protein and other proteins expressed in Bacillus, in a medium with the addition of copper sulfate is directly related to the biosynthesis of monoatomic copper (image No. 4).
For a more detailed acquaintance with the nuances of the study, I recommend that you look at scientists report and Additional materials to him.
Epilogue
In this work, scientists investigated a rather curious organism – a bacterium Bacilluscapable of intracellularly synthesizing monoatomic copper (Cu0) from copper sulfate (CuSOfour). This process, which is natural for bacteria, is extremely difficult to reproduce using even the most modern technologies and techniques. For the production of monoatomic copper, people are forced to use toxic reagents, while bacteria do not need it.
According to the authors of the work, their results provide a better understanding of how such small organisms are able to regulate such complex chemical reactions. In addition, this research could form the basis for the industrial production of atomic copper, which will be devoid of previous disadvantages, but will gain new advantages, such as reduced production costs.
Considering that copper is used in many ways (from antibacterial coating to electronics), these kinds of discoveries really carry considerable potential.
It is also important to note that the bacteria studied may not be the only microorganisms capable of synthesizing monatomic metals, which will find their application in science, technology, and medicine.
Thanks for your attention, stay curious and have a good work week, guys. 🙂
A bit of advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting content? Support us by placing an order or recommending to friends, cloud VPS for developers from $ 4.99, a unique analogue of entry-level servers, which was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server correctly? (options available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Is Dell R730xd 2x cheaper in Maincubes Tier IV data center in Amsterdam? Only here 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 – 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB – From $ 99! Read about How to build the infrastructure of bldg. class with the use of Dell R730xd E5-2650 v4 servers at a cost of 9000 euros for a penny?





